Medical Device Projects
Coronary and cerebrovascular device design, development and manufacturing engineering support

-design and manufacturing development of cardio/ cerebrovascular and peripheral stent implants.

- de novo, restinotic and bifurcated lesion specific devices

-non linear/explicit dynamics based predictions and response modeling.

- pushiable/trackable/flexible delivery with optimal, reliable deployment

- kissing balloon delivery system

-from concept to delivery of successfully tested, working prototypes

-rapid assembly prototyping of functional test units based on end use materials.

- protocol preparation, testing, analysis, reporting

- IDE clinical trial pilot device manufacturing process development

- enhanced catheter flexibility delivers first stent to MCA blockage

- balloon installation via progressive, uniform rotary motion with minimum final aperture sizing required

-50 micron diameter automated stent joining process - pulsed Nd-YAG laser development

- scanning electron micro graph of uniform stent welds

- femtosecond laser based cutting process development of pre-formed hypo tube and polymer based structures
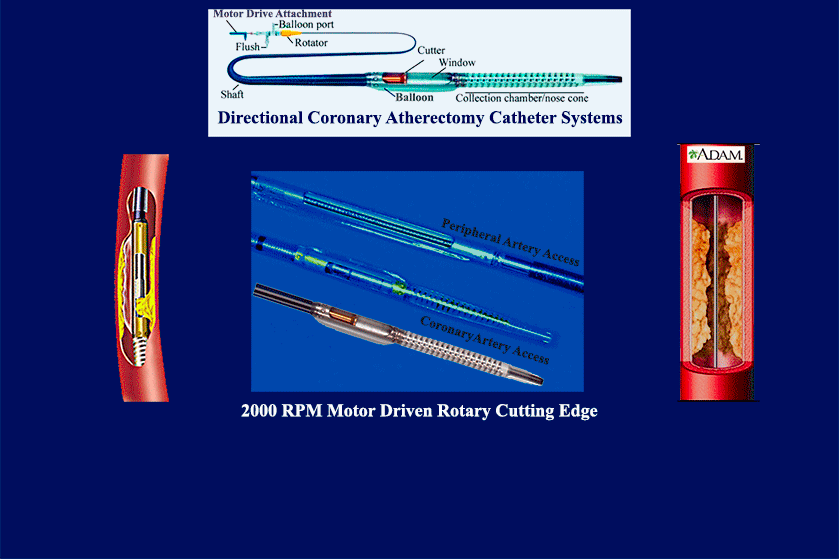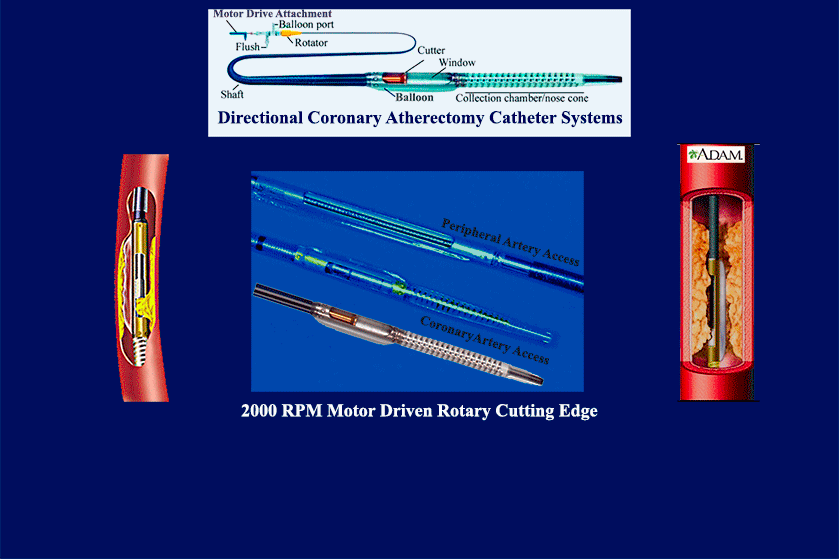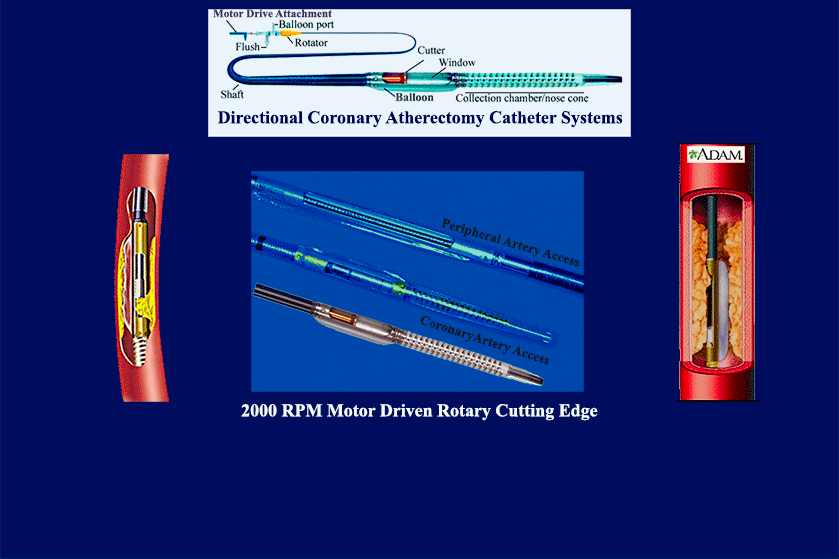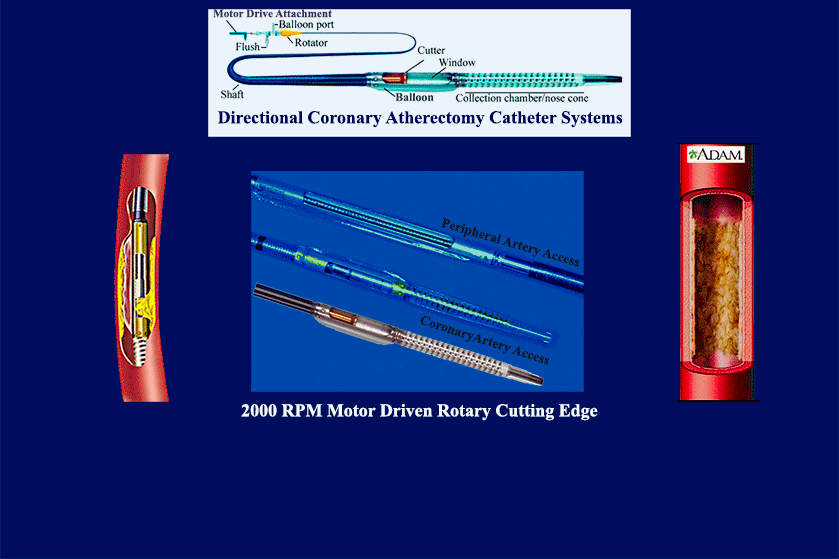
- multiple product & mfg development launches

- flat stainless ribbon braiding of .001 x .003/.001 x .005 inch material for catheters ranging from 5 to 10F

- development/integration/process development

- development/integration/process development

- automated weld positioning/inspection

- multiple stent production cycles with zero tolerance accumulation

-design / fabrication / test / delivery

-progressive rotary stent crimping

- 3 week design / build / test program

- arterial pressure transducer

- photo of board level residue identified by energy dispersive X-ray analysis (EDXA)

- magnified view of wire bond failures (circle areas)

- pressure transducer protection during PCB fab

- 4 day critical design + fabrication turn around cycle

- 512 masks for qualification in 5 days






























